Cell separation from blood
The enrichment of cells and the optimisation of cell separation methods play a decisive role for many researchers. Various approaches exist for sorting different cell types, with the choice of the appropriate method often depending on the cell type or cell subset. One of the faster and more cost-effective methods for separating cells is density gradient centrifugation. In our article"Cell separation and cell separation methods" we have summarised the various options for enriching cells from heterogeneous mixtures, such as blood.
Density of cells as an aid for cell separation
Based on the different densities of the individual blood components, these can be separated by single-stage centrifugation with the appropriate density gradient medium. The technique is based on the fact that particles with different densities have different sedimentation rates during centrifugation. Heavy particles such as erythrocytes sediment quickly, while lighter cells such as thrombocytes sediment more slowly. The following figure shows the distribution of the most important cell populations in blood. Erythrocytes have the highest density and platelets the lowest density.
Distribution of blood cells as a function of density
The following figure (Figure 1) shows the distribution of blood cells depending on their density. Thrombocytes (1) have the lowest density, erythrocytes (2) the highest density. The number of cells per population is also shown. Erythrocytes represent the largest population, platelets the second largest population.
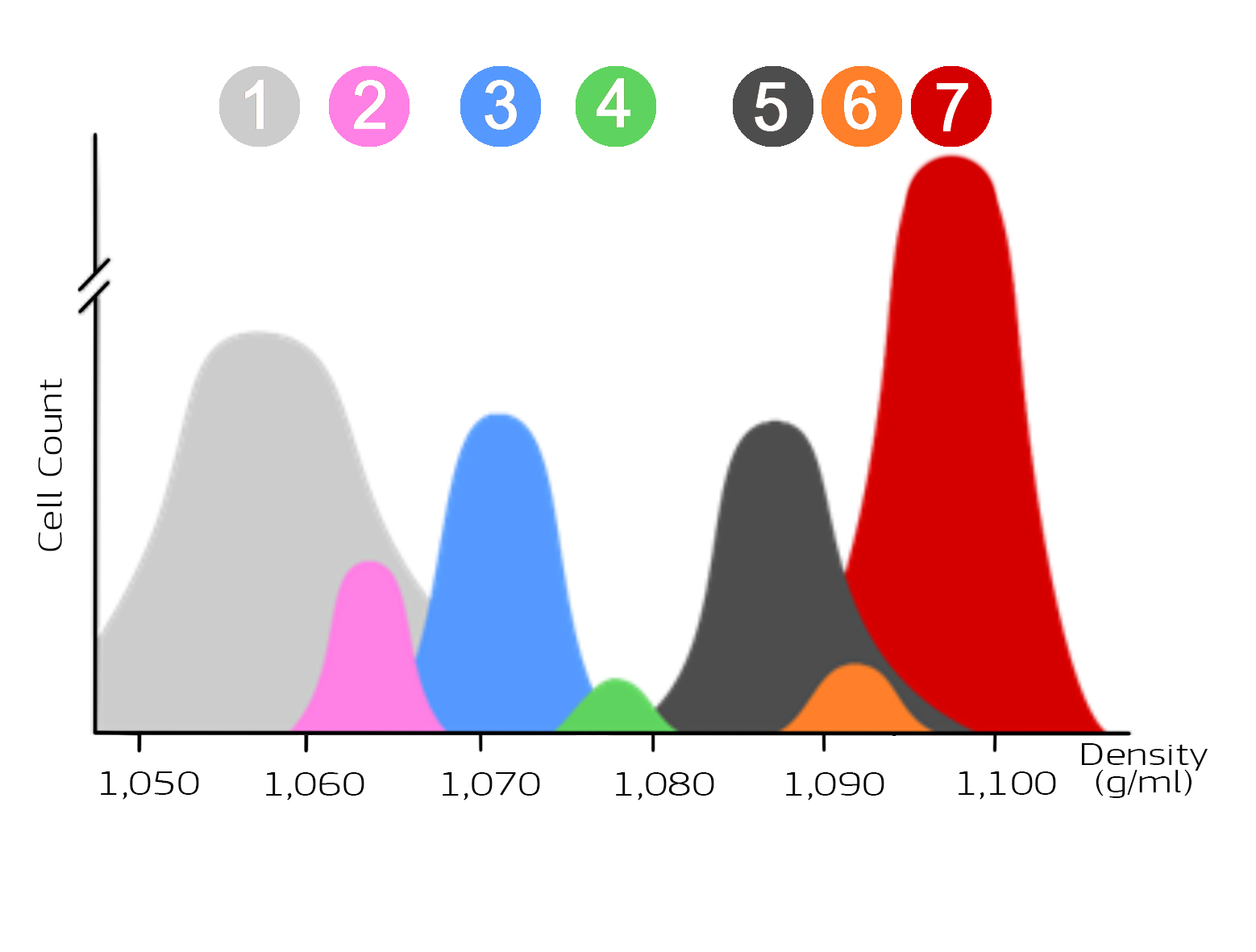
Figure 1: Illustration of the cell populations of blood as a function of density
The cell populations thrombocytes (1), monocytes (2), lymphocytes (3), basophil granulocytes (4), neutrophil granulocytes (5), eosinophil granulocytes (6) and erythrocytes (7) differ in density and cell number. The different densities of the cell populations are used for enrichment with the aid of density gradient media.
Table: Density ranges of blood cells
Typ | Blood Cell | Density (g/ml) |
1 | Thrombocytes | 1,050 - 1,070 |
2 | Monocytes | 1,060 - 1,068 |
3 | Lymphocytes | 1,067 - 1,077 |
4 | Basophilic granulocytes | 1,072 - 1,081 |
5 | Neutrophil granulocyte | 1,079 - 1,098 |
6 | Eosinophil granulocytes | 1,089 - 1,095 |
7 | Erythrocytes | 1,090 - > 1,100 |
Typ | Blood Cell | Density (g/ml) |
1 | Thrombocytes | 1,050 - 1,070 |
2 | Monocytes | 1,060 - 1,068 |
3 | Lymphocytes | 1,067 - 1,077 |
4 | Basophilic granulocytes | 1,072 - 1,081 |
5 | Neutrophils | 1,079 - 1,098 |
6 | Eosinophil granulocytes | 1,089 - 1,095 |
7 | Erythrocytes | 1,090 - > 1,100 |
As can be seen from the figure and the table, the density of the cells or cell populations is a distribution around a mean value. In addition, the densities of the different cell populations overlap. The range as well as the distribution around the mean value are not fixed values. Fluctuations in density can be influenced by various factors. One of the most important factors influencing the density of the cells is the hydration of the cells. Another important factor influencing the density of whole blood cells is the time outside the donor organism.
Use of density gradient media for density gradient centrifugation
With the knowledge of the density of blood cells, it is possible to specifically enrich different cell populations. This involves the use of density gradient media - solutions with a defined density. This density determines the separation limit (cut-off). Cells with a lower density than the cut-off collect as a layer above the medium, while cells with a higher density pass through the separation medium and settle at the bottom of the tube. The following figure shows a typical image for the separation of whole blood with a separation medium for the enrichment ofperipheral blood mononuclear cells - PBMC for short.
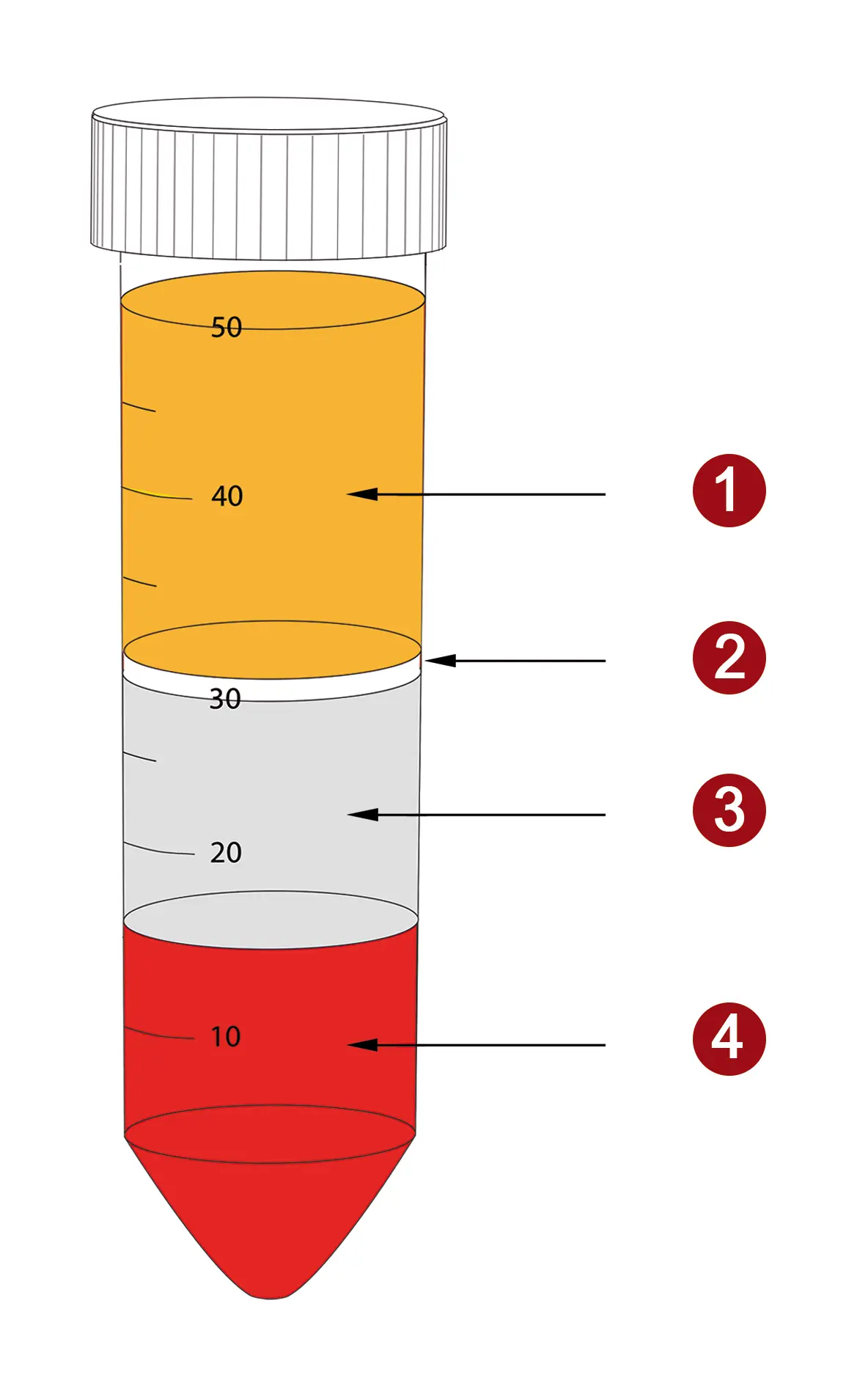
Figure 2: Distribution of layers after density gradient centrifugation of whole blood with PBMC spin medium. PBMC (2) collect between plasma (1) and density medium (3). Erythrocytes, granulocytes and dead cells pass through the medium and are concentrated at the bottom of the tube (4).
Density media for different cell populations
PBMC Spin Medium, density gradient medium

Sterile, ready-to-use solution for the in vitro isolation of human PBMC from fresh sample material
PBMC 24+ Spin Medium, density gradient medium

Sterile, ready-to-use solution for the in vitro isolation of human PBMC from sample material more than 12 hours old
Leuko Spin Medium, density gradient medium

Sterile, ready-to-use solution for the in vitro isolation of all leucocytes from fresh human sample material
Leuko 24+ Spin Medium, density gradient medium

Sterile, ready-to-use solution for the in vitro isolation of all leucocytes from human sample material that is more than 12 hours old
Monocyte spin medium, density gradient medium

Sterile, ready-to-use solution for the in vitro isolation of human monocytes from fresh sample material
PLT Spin Medium, density gradient medium

Sterile, ready-to-use solution for the in vitro isolation of human platelets (PLT) from fresh sample material