Cookie Settings
We use cookies to provide an optimal experience for you. Technically required cookies are used for making shopping possible, statistics are used for anonymized Google Analytics. You can read everything in our updated privacy policy.
Here we are presenting a new approach to colon carcinoma circulating tumor cell (CTC) screening using pluriBead® carrying a tumor-associated EpCAM antibody.
This method is based on a non-magnetic cell separation technology. It does not require any sample pre-treatment. EpCAM-pluriBead® can be added directly to a whole blood sample. The method is also suitable for single cell isolation from different biological fluids. Moreover, its sensitivity can be additionally increased by raising the sample volume. The bound EpCAM-positive colon carcinoma cells can be easily involved in further molecular-genetic experiments that aim to detect of their mutation status. In case of colon carcinoma, K-ras mutation status is a predictive tool of response anticancer therapy. Thus, the new method can be considered as a fast and effective instrument for early cancer diagnostics.
 |
 |
 |
 |
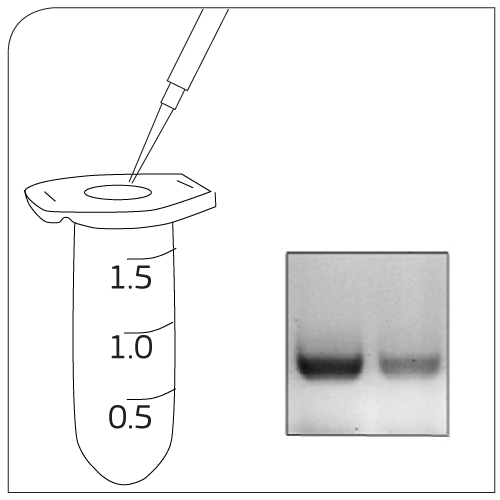 |
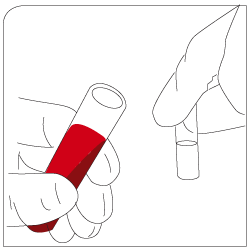
Labeling Add EpCAM-pluriBead® to your sample |
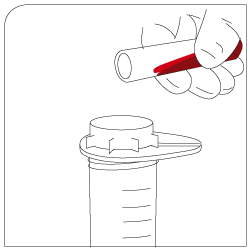
Mixing 30 minutes of gentle incubation (recommended with pluriPlix®). |
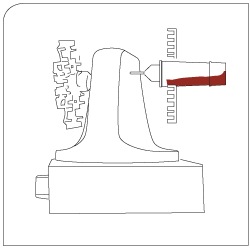
Isolation Captured target cells are separated via appropriate sieves. |
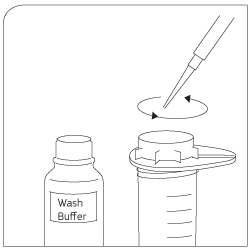
Washing Use wash buffer to clean sieve. Lyse cells with Trizol®. |
CTC Processing Approaches for the study of cancer cells:- RNA/DNA isolation - Cell culture experiments |
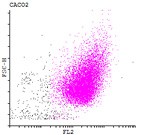
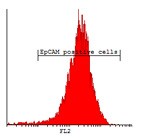
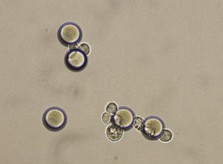
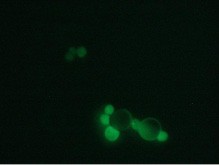

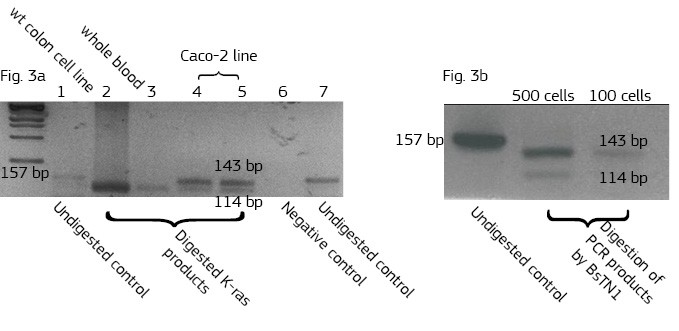

Here we developed a new approach for the detection of circulating colon tumor cells in blood using EpCAM-pluriBead®. Captured cells are suitable for further molecular-genetic screening of specific markers, connecting with tumor formation. The developed “in situ immunobeads pcr” method does not require preliminary RNA/DNA isolation and can effectively save the time of analysis. The further aim of this work is to increase the sensitivity of method raising the sample volume, varying the cell tumor lines and/or antibody specificity.